COVID-19 vaccine(FPP006)
COVID-19 vaccine(FPP006)
- Home
- COVID-19 vaccine(FPP006)
Novel Coronapeptide Vaccine
With the global spread of the novel coronavirus (COVID-19), the development of vaccines against SARS-CoV-2 has progressed at an unprecedented speed. While the vaccines have shown very high efficacy, it has been reported that some people do not wish to be vaccinated due to strong adverse reactions.
It has been reported that neutralizing antibodies decrease in the body after a certain period of time after vaccine administration, and booster vaccinations (additional vaccinations to increase immunity) have been promoted in the U.S., Europe, Japan, and other countries. With a view to regular booster vaccination in the future, the new SARS-CoV-2-derived peptide vaccine is expected to improve on the issues of existing vaccines by (1) extending the duration of immunity, (2) responding to a wide range of mutant strains, and (3) reducing strong adverse reactions.
Toward a universal vaccine unaffected by viral mutations with high efficacy and weak adverse reactions
We are conducting research and development of a peptide vaccine against SARS-CoV-2, based on the platform technology of antibody-inducing peptides, a type of peptide vaccine. While existing vaccines induce immunity using whole viruses or target proteins (mRNA vaccines, DNA vaccines, viral vector vaccines, recombinant proteins and inactivated vaccines, etc.) as antigens, FunPep has selected peptide sequences (epitopes) without mutations for the peptide vaccine against SARS-CoV-2. It is characterized by efficiently inducing immunity, and we are developing the vaccine with the aim of making use of this feature to become a vaccine unaffected by viral mutations with high efficacy and minimal adverse reactions.
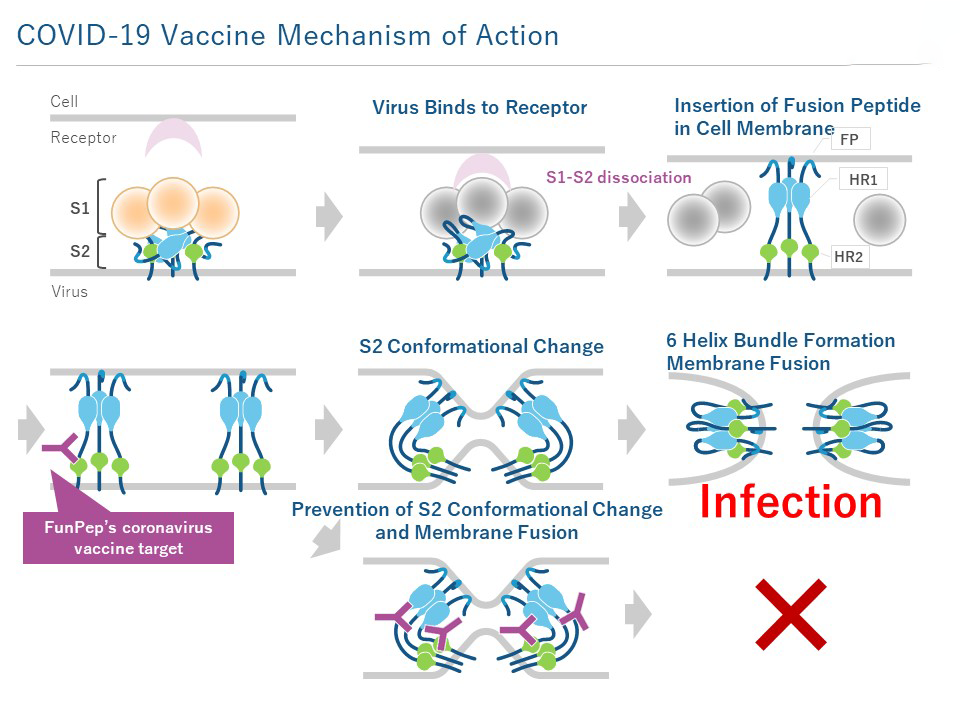
The infection of SARS-CoV-2 virus to human cells is initiated by the Spike protein binding to ACE2 receptor on the surface of cell membrane. The spike protein is composed of two domains: S1, the receptor binding site, and S2, which mediates fusion to the cell to be infected. Once the S1 domain binds to the ACE2 receptor, the S1 domain is released from S2, exposing the S2 domain. When the fusion peptide (FP) in the S2 domain is inserted into the target cell membrane, S2 domain conformational change (6 helix bundles, 6HB formation) occurs, adopting a hairpin structure with three HR2 strands covering the outside of the trimeric HR1 bringing the envelope and target cell membrane adjacent to each other. Fusion occurs between the two types of membranes, and finally the viral genome enters the cytoplasm.
The antibodies induced by our peptide vaccine inhibit the conformational change of the S2 domain (formation of a hairpin structure), thereby inhibiting viral fusion with the cell, or infection. Since this epitope region is conserved among coronaviruses, and since no mutation has been reported in SARS-CoV-2, it is expected to be an effective universal epitope vaccine against new mutant strains.